DANIEL CELL:
* IT IS A PRIMARY CELL.
*IT CANNOT SUPPLY STEADY CURRENT FOR A LONG TIME.
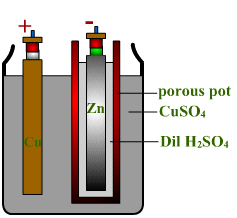
*ANODE - COPPER VESSEL
CATHODE - ZINC ROD DIPPED IN Dil.H2SO4 IN A POROUS POT
ELECTROLYTE - CuSO4 (COPPER SULPHATE) SOLUTION IN WHICH THE POT IS PLACED
EMF - 1.08 V.
*Zn + H2SO4 -----> ZnSO4 + 2H + 2 electrons
WHEN ZINC REACTS WITH Dil.H2SO4 ,Zn IONS AND TWO ELECTRONS ARE GIVEN OUT.
*Zn IONS DIFFUSE THROUGH THE PORES OF THE POROUS POT AND REACTS WITH CuSO4 PRODUCING Cu IONS.
*Zn + CuSO4 -----> ZnSO4 + Cu
*Cu IS DEPOSITED ON THE ANODE.
*WHEN THE CELL IS CONNECTED BY A WIRE ,TWO ELECTRONS ON THE ZINC ROD PASS THROUGH THE EXTERNAL CIRCUIT AND REACH COPPER VESSEL NEUTRALISING Cu.
*THUS CURRENT FLOW FROM Cu TO Zn.
 |
| JOHN DANIEL |
*IT CANNOT SUPPLY STEADY CURRENT FOR A LONG TIME.
*ANODE - COPPER VESSEL
CATHODE - ZINC ROD DIPPED IN Dil.H2SO4 IN A POROUS POT
ELECTROLYTE - CuSO4 (COPPER SULPHATE) SOLUTION IN WHICH THE POT IS PLACED
EMF - 1.08 V.
*Zn + H2SO4 -----> ZnSO4 + 2H + 2 electrons
WHEN ZINC REACTS WITH Dil.H2SO4 ,Zn IONS AND TWO ELECTRONS ARE GIVEN OUT.
*Zn IONS DIFFUSE THROUGH THE PORES OF THE POROUS POT AND REACTS WITH CuSO4 PRODUCING Cu IONS.
*Zn + CuSO4 -----> ZnSO4 + Cu
*Cu IS DEPOSITED ON THE ANODE.
*WHEN THE CELL IS CONNECTED BY A WIRE ,TWO ELECTRONS ON THE ZINC ROD PASS THROUGH THE EXTERNAL CIRCUIT AND REACH COPPER VESSEL NEUTRALISING Cu.
*THUS CURRENT FLOW FROM Cu TO Zn.


It's a wonderful post! I really happy to see this great informative blog. Keep writing more. zink anode Zinc anodes for sale. Order your sacrificial zinc anodes today and save. All size Zimar zinc anodes available for shipment today.
ReplyDelete